Informed Consent
You’re probably familiar with the phrase “informed consent” if you’ve participated in any medical research studies or procedures. But did you know that informed consent actually starts much earlier in the development process for new drugs and medical interventions? Informed consent consulting helps researchers design ethical clinical trials and get vital treatments to patients faster. This is what in any case we can’t neglect the importance of informed consent if we want. It’s considered as the base of everything.
As a participant in a clinical trial, you expect researchers to disclose all the potential risks and benefits before you sign on the dotted line. But researchers can’t know all the risks and benefits of an unproven intervention before testing it on humans. That’s where informed consent consultants come in. They help identify the kinds of information that different groups of participants will need to give their meaningful consent.
Informed consent consulting leads to better informed participants, more useful data, and ultimately, safer and more effective new treatments. The next time you or a loved one considers participating in a clinical trial, you’ll be glad these behind the scenes experts helped pave the way. Informed consent may not be the most exciting part of medical research, but it’s absolutely crucial for ethical progress.
Why Informed Consent Essential In Clinical Trials ?
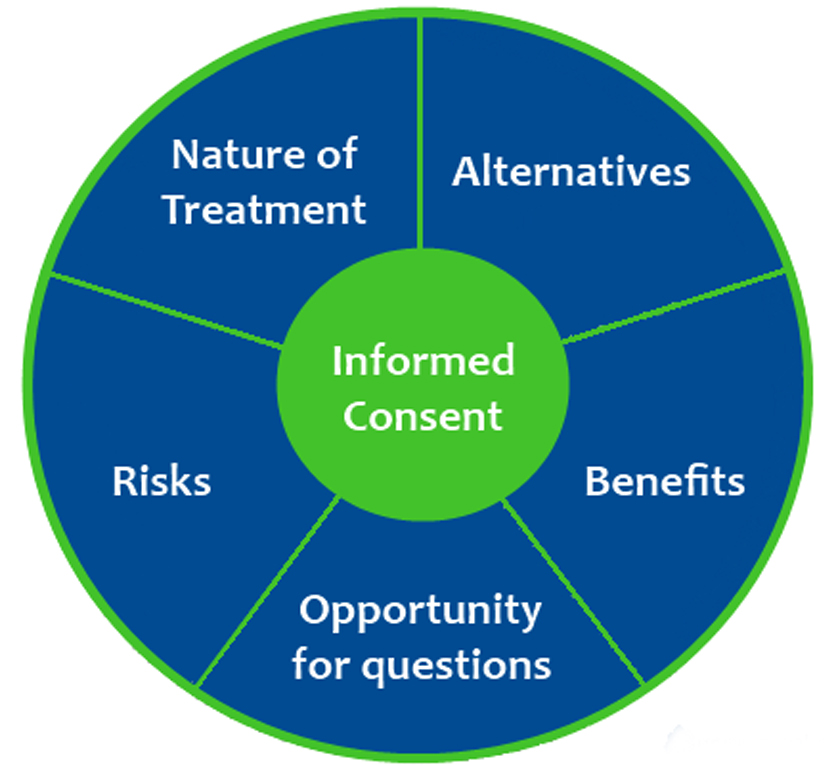
Informed consent is crucial for conducting ethical clinical trials. As a participant, you have rights and your consent matters.Why is informed consent so important? For one, it protects your autonomy and ensures your voluntary participation. You have the right to know exactly what the study involves before agreeing to take part. The researchers must disclose everything from the purpose and duration of the trial to any potential risks or benefits.
Once you have all the details, you can make an educated decision about whether participating is right for you. Your consent also allows the research team to collect and use your information and biospecimens. But you can withdraw at any time if you change your mind.
An informed consent form outlines all these details and more. A member of the research team will go over everything with you and answer any questions you may have before asking you to sign. Informed consent empowers you as a participant. And empowered participants are essential for ethical research that aims to benefit us all.
How Informed Consent Experts Support Drug Development?
Informed consent help drug development consulting and understand the specific regulations and requirements around informed consent that apply to their clinical trial. They can interpret guidelines from agencies like the FDA and others.With frequent changes to regulations, staying on top of requirements is challenging and drug safety experts help ensure remain compliant.
For every clinical trial, informed consent forms and an informed consent process must be developed to match the trial’s objectives, risks and benefits. Informed consent consultants work with well to create consent forms that are complete, clear and concise, using language appropriate for the target patient population. They also help determine the most effective process for obtaining informed consent, whether it involves paper forms, or a combination.
Informed consent is an ongoing process that requires continuous support and monitoring. Consultants provide support for any questions or issues that arise related to informed consent during a clinical trial. They also monitor key metrics around comprehension and satisfaction to identify any need for improvements to forms or processes.
Conclusion
Therefore, informed consent consulting services are extremely valuable for pharmaceutical companies and contract research organizations conducting clinical trials. They help ensure regulatory compliance, enhance participant understanding and experience, and ultimately speed up the drug development process.
You may also like
-
STD Clinic Dubai: Confidential & Professional STD Testing
-
Buy Shilajit UK Guide to Finding Authentic Himalayan Shilajit
-
Buy Shilajit Online: A Modern Path to Ancient Wellness
-
The Best Shilajit Brands: A Complete Guide to Quality, Purity & Performance
-
Unlocking Nature’s Secret: The Best Place to Buy Shilajit